Overall goal
Our main focus is how adult stem cells make their correct cellular lineage in space and time to replace the exact number and type of cells lost to physiological turnover (homeostasis) or injury (regeneration). The freshwater planarian, Schmidtea mediterranea, offers a unique system to study stem cell lineage production in adult animals in vivo. Planarians are in the phylum Platyhelminthes and are members of the super-phylum Lophotrochozoa. This is advantageous and will also lend a fresh view on adult stem cell biology and cancer development.
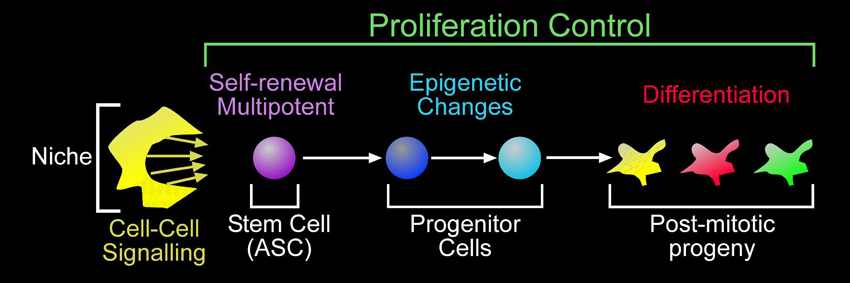
Proliferation control in adult stem cell lineages
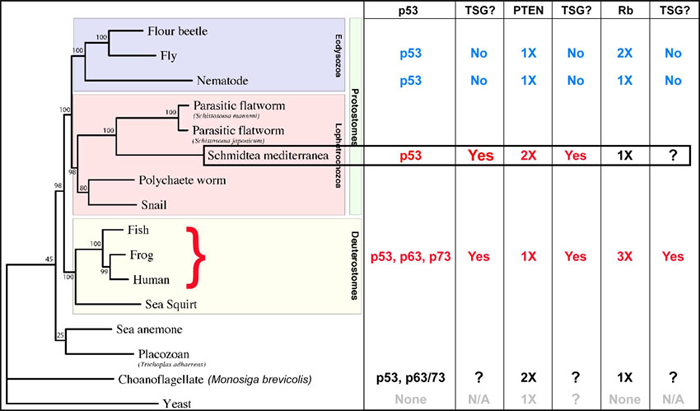
Planarians give us unprecedented access to study and manipulate an entire population of adult stem cells in vivo and, at the same time, assay changes in lineage development. To begin to understand the proliferation control mechanisms used by planarians, we decided to test the three main tumour suppressor pathways that are mutated in human cancers: p53, PTEN, and Retinoblastoma. These genes were also chosen because the Drosophila and C. elegans homologs do not function as bona fide tumour suppressors and there have never been functional studies of these pathways in an animal belonging to the Lophotrochozoan super-phylum. Thus far, we have shown that PTEN and p53 function to limit proliferation in stem cell lineages, similar to their vertebrate counterparts. Planarians are the first invertebrate lab model to have tumour suppressor function for homologs of these human cancer genes which suggests that they will be an invaluable disease model to discover novel stem cell regulators.
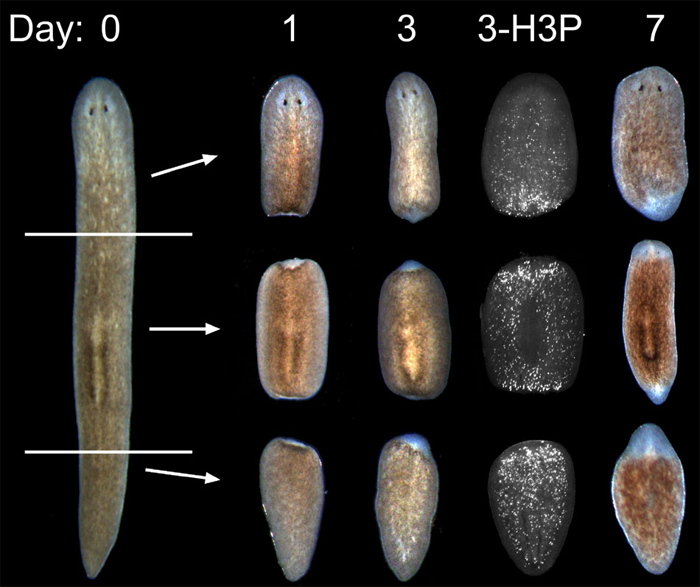
Regeneration
Planarians are masters of regeneration where an amputated fragment as small as 1/279th of the original worm can regenerate an entire adult. From the perspective of the stem cells, several interesting changes occur. First, the strict proliferation control observed in intact adults must be temporarily relieved in order for the stem cells to hyper-proliferate and generate the new tissue that has been lost to injury. Second, there must be a spatial re-patterning of the stem cell lineages to precisely replace the missing body regions. For example, when a small tail fragment is amputated, the stem cells in the tail must now make a new anterior, eyes, and brain de novo– even though those cells have never been required to make those differentiated cell types before (because they were located in the tail). How the stem cells reacquire spatial positioning, and then how their lineages change to make the missing differentiated cell types is a mechanism we are trying to understand.
Evo-devo
Owing to their beneficial phylogenetic position, planarians offer a unique opportunity to functionally study the evolution of developmental pathways. In addition, the asexual species we work on no longer has embryogenesis. Thus, it remains an open question as to how planarians use well-known developmental genes in adult animals, and what this might be able to tell us about their evolutionary history and body patterning. We are trying to understand how planarians have come to have a bodyplan that is not obviously segmented and only has a single, ventral, body opening. To this end, we are looking at planarian homologs of critical developmental pathways for these patterning events from fly and vertebrates.
